Abstract
Background: Pembrolizumab, the high-affinity antibody against programmed death 1 (PD-1), demonstrated effective antitumor activity and tolerable safety in patients with relapsed or refractory classical Hodgkin lymphoma (RRcHL) in KEYNOTE-087. The 2014 Lugano classification (Cheson et al. J Clin Oncol . 2014;32:3059-67) for lymphoma provides recommendations designed to improve response evaluation compared with the 2007 Revised Response Criteria for Malignant Lymphoma (Cheson et al. J Clin Oncol. 2007;25:579-86). The Lugano classification assigns more weight to changes seen on positron emission tomography (PET)-computed tomography and introduces the 5-point scale for PET assessment. Whereas Cheson 2007 advised using either an increase in the sum of products of diameters or growth of individual lesions to detect progressive disease (PD), Lugano recognizes that growth of individual lesions is always reflected in the growth of the aggregate value, so the sum need not be evaluated when looking for PD. Bone marrow biopsy is not indicated for routine staging (instead of biopsy for staging, per Cheson 2007), and for fluorodeoxyglucose-avid lymphomas, PET can replace bone marrow biopsy. A comparison of response by Cheson 2007 and Lugano 2014 in KEYNOTE-087 is presented.
Methods: The phase 2 multicenter, single-arm KEYNOTE-087 (ClinicalTrials.gov, NCT02453594) study examined the safety and efficacy of pembrolizumab in 3 cohorts of patients with RRcHL. Cohort 1 included patients whose disease progressed after autologous stem cell transplantation (ASCT) and subsequent brentuximab vedotin (BV); cohort 2, after salvage chemotherapy and BV; cohort 3, after ASCT but who had not received BV after transplantation. Patients received pembrolizumab 200 mg intravenously every 3 weeks for up to 24 months or until confirmed disease progression, unacceptable toxicity, or investigator or patient decision for withdrawal. Response was assessed every 12 weeks by blinded independent central review (BICR) per the Cheson 2007 criteria and Lugano 2014 classification, with the same reviewers performing both assessments sequentially. Primary end points were safety and objective response rate (ORR) by BICR per Cheson 2007. Key secondary end points included ORR by investigator assessment per Cheson 2007; ORR by BICR per the 2014 Lugano classification; complete response (CR) rate by BICR and investigator review per Cheson 2007 criteria; and CR rate by BICR per the 2014 Lugano classification. All patients who received ≥1 dose of pembrolizumab were included in the analyses. Results from the database cutoff of March 21, 2017, are presented.
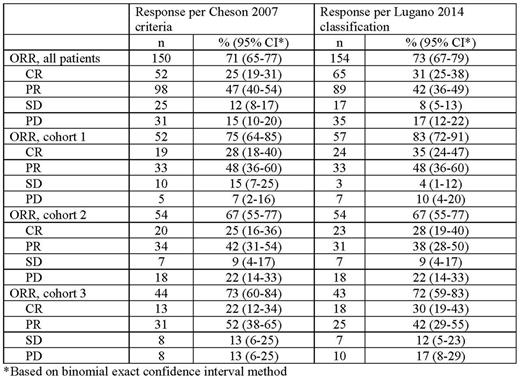
Results: This study enrolled 210 patients with RRcHL. Baseline characteristics have been reported previously (Chen et al. J Clin Oncol. 2017;35:2125-32). Median follow-up was 15.9 months (range, 1.0-20.9 months). ORR by BICR per Cheson (71%) criteria was similar to that by Lugano classification (73%) (Table). CR rates were slightly higher (25% vs 31%, respectively) and PR rates were slightly lower (47% vs 42%, respectively). ORR by cohort per Cheson was also comparable to that per Lugano classification (cohort 1, 75% and 83%, respectively; cohort 2, 67% for each; cohort 3, 73% and 72%, respectively). Of 150 responders per Cheson, 139 (93%) were responders and 11 (7%) nonresponders per Lugano classification; of 60 nonresponders per Cheson, 45 (75%) were nonresponders and 15 (25%) were responders per Lugano classification. There was 88% agreement between the ORR rates per Cheson 2007 and those per Lugano classification.
Conclusions: Pembrolizumab monotherapy demonstrated similarly high ORR rates and higher CR rates across all 3 cohorts of patients with heavily pretreated RRcHL by the Lugano 2014 classification than by the Cheson 2007 criteria.
Moskowitz: Pharmacyclics: Research Funding; Celgene: Consultancy; Merck: Consultancy, Research Funding; Seattle Genetics: Consultancy, Other: Ad Board, Research Funding; Genentech BioOncology: Consultancy. Chen: Genentech: Speakers Bureau; Affimed: Research Funding; Merck: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Research Funding; Pfizer: Consultancy; Pharmacyclics: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding, Speakers Bureau. Armand: Infinity: Consultancy; Merck: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Affimed: Research Funding; Pfizer: Research Funding. Zinzani: Celgene, Roche, Janssen, Gilead, Takeda, BMS, MSD, Servier, Sandoz, Mundipharma: Honoraria; Celgene, Janssen, Gilead, Roche, Takeda, BMS, MSD, Sandoz, Servier, Mundipharma: Speakers Bureau; Merck: Consultancy, Other: Advisory board. Goldmacher: Merck: Employment. Lin: Merck: Employment. Nahar: Merck: Employment. Balakumaran: Merck: Employment, Equity Ownership. Salles: Amgen: Consultancy, Other: Advisory board; Bristol-Myers Squibb: Consultancy, Other: Advisory board; Celgene: Consultancy, Other: Advisory board; Gilead: Consultancy, Other: Advisory board; Janssen: Consultancy, Other: Advisory board; Kite: Consultancy, Other: Advisory board; Merck: Consultancy, Other: Advisory board; Morphosys: Consultancy, Other: Advisory board; Novartis: Consultancy, Other: Advisory board; Roche: Consultancy, Other: Advisory board, Research Funding; Servier: Consultancy, Other: Advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal